Answer:
The major organic product is 2,2-dibromoheptane
Step-by-step explanation:
At first, 1-heptyne is protonated by 1-equivalent HBr and produces a stable
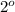 carbocation followed by nucleophilic attack by bromide ion.
carbocation followed by nucleophilic attack by bromide ion.
Then another equivalent of HBr adds on double bond to produce another
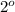 carbocation which is highly stable due to electron donating resonating effect of Br atom (+R effect). Thus a consequent nucleophilic attack onto that carbocation leads to formation of 2,2-dibromoheptane as a major product.
carbocation which is highly stable due to electron donating resonating effect of Br atom (+R effect). Thus a consequent nucleophilic attack onto that carbocation leads to formation of 2,2-dibromoheptane as a major product.
Detailed mechanism has been attached below.