Step-by-step explanation:
It is known that acetic acid is a weak acid. It's equilibrium of dissociation will be represented as follows.
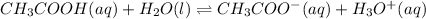
On the other hand, sodium acetate (
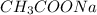 ) is a salt of weak acid, that is,
) is a salt of weak acid, that is,
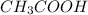 and strong base, that is, NaOH. Therefore, aqueous solution of sodium acetate will be basic in nature.
and strong base, that is, NaOH. Therefore, aqueous solution of sodium acetate will be basic in nature.
Since, acetic acid is a weak acid but still it is an acid. So, when methyl orange is added in a solution of acetic acid then it given a reddish-orange color because of its acidity.
When sodium acetate is mixed into this solution then it will dissociate as follows.
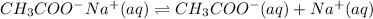
As both solutions are liberating acetate ion upon dissociation. Hence, it is the common ion.
So, when more acetate ions will increase from dissociation of sodium acetate the according to Le Chatelier's principle the equilibrium will shift on left side.
As a result, there will be decrease in the concentration of hydronium ions. As a result, there will be increase in the pH of the system.
Hence, color of methyl orange will change from reddish orange to yellow. This shift in equilibrium is due to the common ion which is
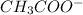 ion.
ion.