Answer: The amount of carbon sulfide prepared is 859.1724 grams.
Step-by-step explanation:
To calculate the concentration of
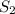 , we use the equation:
, we use the equation:
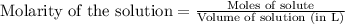 .....(1)
.....(1)
We are given:
Moles of
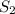 = 12.5 moles
= 12.5 moles
Volume of solution = 5.3 L
Putting values in above equation, we get:
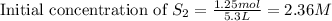
For the reaction of sulfur and carbon, the equation follows:
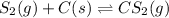
At
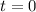 2.36
2.36
At
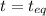 2.36 - x x
2.36 - x x
- The expression for equilibrium constant for the above reaction follows:
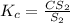
We are given:

![[CS_2]=x](https://img.qammunity.org/2020/formulas/chemistry/high-school/6ygdhfb7gq5zun61y9tbepifil4lqf67y1.png)
![[S_2]=2.36-x](https://img.qammunity.org/2020/formulas/chemistry/high-school/j0ytg3jj9jphas64knzqa10hfguvd8sflu.png)
Putting values in above equation, we get:
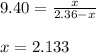
- Now, calculating the moles of carbon sulfide using equation 1, we get:
Molarity of
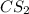 = 2.133 M
= 2.133 M
Volume of solution = 5.30 L
Putting values in equation 1, we get:
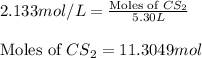
- To calculate the mass of carbon sulfide, we use the equation:

Moles of carbon sulfide = 11.3049 mol
Molar mass of carbon sulfide = 76 g/mol
Putting values in above equation, we get:
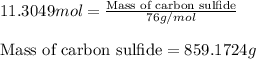
Hence, the amount of carbon sulfide prepared is 859.1724 grams.