Answer:
3.75 grams of methane
Step-by-step explanation:
Given parameters:
Mass of oxygen gas = 5g
Mass of CH₄ = 5g
Unknown parameters:
Amount of excess reactant = ?
Solution
In order to find the amount of leftover reactant we need to know the reactant that is in excess and in limited supply. The amount of reactant in short supply will determine the extent of the reaction.
Now, we need to write the balanced reaction equation:
CH₄ + 2O₂ → CO₂ + 2H₂O
We first find the number of moles of the reactants.
Number of moles of methane =
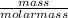
Number of moles of methane =
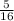 = 0.3125mole
= 0.3125mole
Number of moles of oxygen =
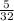 = 0.156mole
= 0.156mole
From the equation:
1 mole of methane reacts with 2 moles of oxygen gas
x moles of methane reacts with 0.156moles of oxygen gas
2x = 0.156
x = 0.078moles
We see that the methane is in excess and oxygen gas is in short supply in the reaction.
The amount of excess methane = 0.3125 - 0.078 = 0.2345moles
Now we convert it to mass:
Mass = number of moles x molar mass = 0.2345 x 16 = 3.75grams