Answer:
Rate of the reaction is 0.2593 M/s
-0.5186 M/s is the rate of the loss of ozone.
Step-by-step explanation:
The rate of the reaction is defined as change in any one of the concentration of reactant or product per unit time.
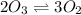
Rate of formation of oxygen :
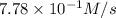
Rate of the reaction(R) =
![(-1)/(2)(d[O_3])/(dt)=(1)/(3)(d[O_2])/(dt)](https://img.qammunity.org/2020/formulas/chemistry/college/u40gg36tvm02v2ak4clg3aid60n7ntrof7.png)
![R=(1)/(3)(d[O_2])/(dt)](https://img.qammunity.org/2020/formulas/chemistry/college/hdsm44funwvxxijwa50mot32n7qhkct3vr.png)
Rate of formation of oxygen=3 × (R)
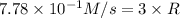
Rate of the reaction(R):
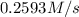
Rate of the reaction is 0.2593 M/s
Rate of disappearance of the ozone:
![R=-(1)/(2)(d[O_3])/(dt)](https://img.qammunity.org/2020/formulas/chemistry/college/4k9ymv5qo5wlhtx4kt914nfq47m4nybi0m.png)
![(d[O_3])/(dt)=-2* R=-2* 0.2593* M/s=-0.5186M/s](https://img.qammunity.org/2020/formulas/chemistry/college/ttrx3cmd1r3yfx8oejspcy3xsmwszarsvo.png)
-0.5186 M/s is the rate of the loss of ozone.