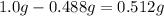Answer:
It could be extracted 0.512 g of solute
Step-by-step explanation:
The equation that relates the
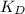 and the volumes of organic and aqueous phases is:
and the volumes of organic and aqueous phases is:
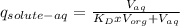
Where q_{solute-aq} refers to the fraction of solute remaining in the aqueous phase, V_{aq} is the aqueos phase volume, V_{org} is the organic phase volume and K_{D} is the partition coefficient of the solute in the solvents.
Moreover,for the three consecutive extractions of the same volume of organic phase we can write:
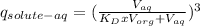
So, plugging the values given into the equation we get:
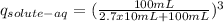
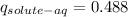
The result obtained indicates that a fraction of 0.488 of solute remains in the aqueous phase.
Taking in account that the fraction formula is:
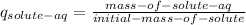
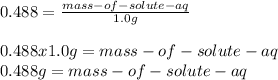
Finally we substract the solute in the aqueous phase form the initial to get the amount in the organic phase: