Answer:
Enthalpy change = -44.12 kJ
Step-by-step explanation:
Given:
ΔH°f(C2H6O(l)) = -277.69 kj/mol
ΔH°f(C2H4(g)) = 52.26 kj/mol
ΔH°f(H2O) = -285.83 kj/mol
To determine:
Enthalpy change for the formation of C2H6O
Calculation:
The given reaction is:
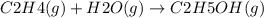
The enthalpy change for the reaction is given as;
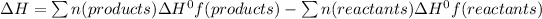
where n(products) and n(reactants) are the moles of products and reactants
Substituting the appropriate values for n and ΔH°f:
![\Delta H = 1\Delta H^(0)f(C2H6O)-[1\Delta H^(0)f(C2H4)+1\Delta H^(0)f(H2O)]](https://img.qammunity.org/2020/formulas/chemistry/college/4m9npt9fw7to6atfrfoy2s5xx4udgt5gnm.png)
ΔH = -277.69-(52.26-285.83) = -44.12 KJ