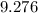Answer:
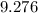
Step-by-step explanation:
As we know that one kilo joules is equal to
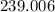 gram calories
gram calories
Given ,
Energy required by thermal decomposition of rail car load of limestone to lime and carbon dioxide is equal to
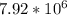 Kilo joules
Kilo joules
This kilo joules of energy is converted into calories and is equal to
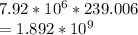
On taking the log of this value, we get -
log (
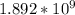 )
)
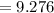
Hence, the answer is