Answer: k [
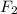 ]
]
![[Cl_(2)O]^(2)](https://img.qammunity.org/2020/formulas/chemistry/college/7aiod46wpg4nkjtvbcdfzrhkuuldaw8go8.png)
Explanation: The given reaction is -
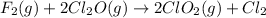
The rate law of the reaction is the written as the concentration of the reactant species rest to the power of its stiochiomeric coefficient.
Thus the rate law of the given reaction can be written as -
Rate = k [
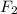 ]
]
![[Cl_(2)O]^(2)](https://img.qammunity.org/2020/formulas/chemistry/college/7aiod46wpg4nkjtvbcdfzrhkuuldaw8go8.png)
Rate law usually is determined from the slowest step of the reaction.