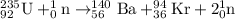Answer: a)
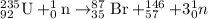
b)
Step-by-step explanation:
A nuclear fission reaction is defined as the reaction in which a heavy nucleus splits into small nuclei along with release of energy.
a) The given reaction is
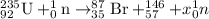
Now, as the mass on both reactant and product side must be equal:
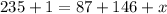
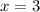
Thus three neutrons are produced and nuclear equation will be:
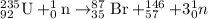
b) For the another fission reaction:
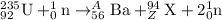
To calculate A:
Total mass on reactant side = total mass on product side
235 + 1 = A + 94 + 2
A = 140
To calculate Z:
Total atomic number on reactant side = total atomic number on product side
92 + 0 = 56 + Z + 0
Z = 36
As Krypton has atomic number of 36,Thus the nuclear equation will be :