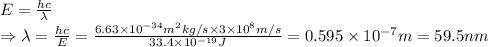Answer:
5.95 nm to 33.6 nm
Step-by-step explanation:
Energy of a single photon can be written as:
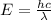
where, h is the Planck's constant, c is the speed of light and λ is the wavelength of light.
Consider the lowest energy of an electron that can break a DNA = 3.45 eV
1 eV = 1.6 ×10⁻¹⁹ J
⇒3.45 eV = 5.52×10⁻¹⁹ J
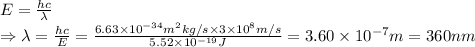
Consider the highest energy of an electron that can break a DNA = 20.9 eV
1 eV = 1.6 ×10⁻¹⁹ J
⇒20.9 eV = 33.4×10⁻¹⁹ J