Answer:
There will be no change since Qc = Kc
Step-by-step explanation:
Given:
[CO] = 0.0360 M
[H2] = 0.0450 M
[H2O] = 0.0200 M
[CH4] = 0.0310 M
Kc = 189
To determine:
The direction in which the given reaction will occur
Calculation:
The given reaction is:
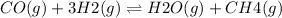
The relation between the equilibrium constant Kc and the reaction quotient Qc determines the reaction direction
![Qc = ([Products])/([Reactants) \\\\For\ the\ given\ reaction\\\\Qc = ([H2O][CH4])/([CO][H2]^(3) ) -----(1)](https://img.qammunity.org/2020/formulas/chemistry/high-school/twhjk2mtuubwe8b4dhn5d6fr842xo03a43.png)
If Qc > Kc reaction will shift to the left
If Qc < Kc reaction will shift to the right
If Qc = Kc there will be no shift
Based on equation (1), for the given reaction:
![Qc= ([0.0200][0.0310])/([0.0360][0.0450]^(3) ) =189](https://img.qammunity.org/2020/formulas/chemistry/high-school/s3jc9hwf7aerpyq8useolzv3n5chz3i1sv.png)
Since Qc=Kc, there will be no change in the reaction direction and the equilibrium will be retained