Answer: The standard reduction potential of X is -1.20 V
Step-by-step explanation:
For the given chemical equation:
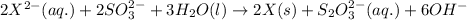
The half reaction follows:
Oxidation half reaction:
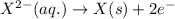 ( × 2 )
( × 2 )
Reduction half reaction:
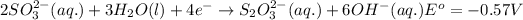
Substance getting oxidized always act as anode and the one getting reduced always act as cathode.
To calculate the
 of the reaction, we use the equation:
of the reaction, we use the equation:
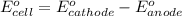
We are given:
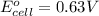
Putting values in above equation, we get:
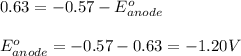
Hence, the standard reduction potential of X is -1.20 V