Answer:

Step-by-step explanation:
1. Define Formula
The compound is carbon tetrabromide. The tetra indicates 4 atoms of bromine.
- Carbon (C)+ 4 Bromine (Br) = CBr₄
2. Find Molar Mass
There are 2 elements in this compound: carbon and bromine. Use the Periodic Table to find the molar masses of these elements.
- Carbon (C): 12.011 g/mol
- Bromine (Br): 79.90 g/mol
The molar mass is based on the number of atoms in the compound. The compound has 1 atom of carbon and 4 atoms of bromine.
CBr₄= 1(12.011 g/mol) + 4(79.90 g/mol)
= 12.011 g/mol+319.60 g/mol = 331.611 g/mol
3. Convert Grams to Moles
We want to convert 675 grams to moles. We should use the molar mass as a fraction.
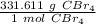
Multiply by the given number of grams.
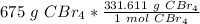
Flip the fraction so the grams of CBr₄ will cancel.
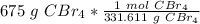 =
=
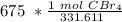
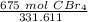 =
=
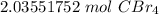
4.Round
The original measurement, 675 grams has 3 significant figures (6,7 and 5), so our answer must have the same.
For the answer we found, 3 sig figs is the hundredth place.
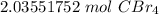
The 5 in the thousandth place tells us to round the 3 to a 4.
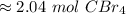
There are about 2.04 moles of carbon tetrabromide in 675 grams.