Step-by-step explanation:
It is given that solvent 1 is benzene and solvent 2 is water. Value of
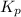 = 2.7.
= 2.7.
Volume of solvent 1 (
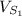 ) = 100 mL.
) = 100 mL.
Volume of solvent 2 (
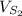 ) = 10 mL.
) = 10 mL.
Therefore, calculate the remaining fraction as follows.
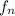 =
=
![[1 + K_(p)(\frac{V_{S_(2)}}{V_{S_(1)}})]^(-n)](https://img.qammunity.org/2020/formulas/chemistry/college/pdcvw4ysccaabcygz2v4sejoupbzhyp1m3.png)
=
![[1 + 2.7((10)/(100))]^(-3)](https://img.qammunity.org/2020/formulas/chemistry/college/unudcwpkrxpdq2o57pse7p9fragf88ct4g.png)
=
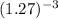
= 0.488
Since, mass of compound is given as 1 gram.
So, reamining extract will be 1 -
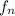 = 1 - 0.488 = 0.512.
= 1 - 0.488 = 0.512.
Thus, we can conclude that the weight of compound extracted is 0.512.