Answer: C) A solution that is 0.10 M
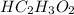 and 0.10 M
and 0.10 M
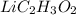 .
.
Explanation: A buffer solution is a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid.
Choice A has equal concentrations(0.10M) of HI and ammonium ion but its not a buffer solution since it has strong acid HI and ammonium ion is also a weak acid.
Choice B has equal concentrations(0.10M) of a NaOH(strong base) and KOH(strong base). Both are strong bases and so its not a buffer solution.
Choice D also has equal concentrations(0.10M) of HBr and potassium acetate. HBr is a strong acid and so this solution is not a buffer solution.
Only choice C is correct as it has acetic acid(weak acid) and a salt (potassium acetate) of its conjugate base.