Answer: The mass of solution having 768 mg of KCN is 426.66 grams.
Step-by-step explanation:
We are given:
0.180 mass % of KCN solution.
0.180 %(m/m) KCN solution means that 0.180 grams of KCN is present in 100 gram of solution.
To calculate the mass of solution having 768 mg of KCN or 0.786 g of KCN (Conversion factor: 1 g = 1000 mg)
Using unitary method:
If 0.180 grams of KCN is present in 100 g of solution.
So, 0.768 grams of KCN will be present in =
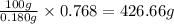 of solution.
of solution.
Hence, the mass of solution having 768 mg of KCN is 426.66 grams.