Answer:
15.01 Liters
Step-by-step explanation:
T₁ = Initial temperature = 25°C = 298.15 K
T₂ = Final temperature = 100°C = 373.15 K
V₁ = Initial volume = 12 mL
Here, pressure is constant so we apply Charles Law
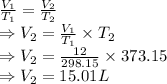
∴ Final volume at 100°C is 15.01 Liters.