Answer: Before addition of HCl, the pH is 3.70 and after addition of HCl, the pH is 3.64 .
Explanation: The pH of the buffer solution is calculated by using Handerson equation:
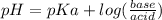
Let's calculate the moles of acid and base(salt) present in the original buffer.
mL are converted to L and then multiplied by molarity to get the moles.
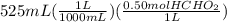
=
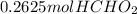
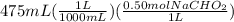
=
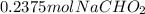
Total volume of the buffer solution = 0.525 L + 0.475 L = 1.00 L
Since, the total volume is 1.00 L, concentration of base will be 0.2375 M and the concentration of acid will be 0.2625 M.
pKa for formic acid is 3.74. Let's plug in the values in the equation and calculate the pH of the original buffer.
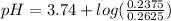
pH = 3.74 - 0.04
pH = 3.70
Now, we add 8.6 mL of 0.15 M HCl acid to 85 mL of the buffer. Let's calculate the moles of acid and base in 85 mL of the buffer.
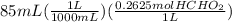
=
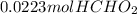
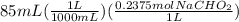
=
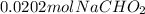
Now, let's calculate the moles of HCl added to the buffer.
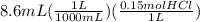
= 0.00129 mol HCl
This added HCl reacts with base(sodium formate) and formic acid is produced.
So, 0.00129 moles of HCl will react with 0.00129 moles of sodium formate to produce 0.00129 moles of formic acid. We can write formate ion in place of sodium formate and hydrogen ion in place of HCl. The equation would be:
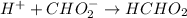
moles of base after reaction with HCl = 0.0202 mol - 0.00129 mol = 0.01891 mol
moles of acid after addition of HCl = 0.0223 mol + 0.00129 mol = 0.02359 mol
Let's plug in the values again in the Handerson equation and calculate the pH:
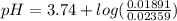
pH = 3.74 - 0.096
pH = 3.64