Answer:
37 mmol of acetate need to add to this solution.
Step-by-step explanation:
Acetic acid is an weak acid. According to Henderson-Hasselbalch equation for a buffer consist of weak acid (acetic acid) and its conjugate base (acetate)-
![pH=pK_(a)(acetic acid)+log[(mmol of CH_(3)COO^(-))/(mmol of CH_(3)COOH )]](https://img.qammunity.org/2020/formulas/chemistry/college/d85qebrly5iwwhesft3au1d11mhh5mpsxe.png)
Here pH is 5.31,
 (acetic acid) is 4.74 and number of mmol of acetic acid is 10 mmol.
(acetic acid) is 4.74 and number of mmol of acetic acid is 10 mmol.
Plug in all the values in the above equation:
![5.31=4.74+log[(mmol of CH_(3)COO^(-))/(10)]](https://img.qammunity.org/2020/formulas/chemistry/college/uc156vgdxihl1gfc1uo38bzwnvnv0hkmf7.png)
or, mmol of
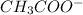 = 37
= 37
So 37 mmol of acetate need to add to this solution.