Answer : The Lewis structure for the given molecule is shown below.
There are 18 sigma
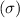 bonds and 4 pi
bonds and 4 pi
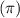 bonds.
bonds.
Explanation :
Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. The number of valence electrons are shown by 'dot'.
The given molecule is,
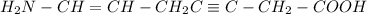
As we know that nitrogen has '5' valance electrons, carbon has '4' valence electrons, hydrogen has '1' valance electron and oxygen has '6' valence electrons.
Therefore, the total number of valence electrons in given molecule = 5 + 7(6) + 9(1) + 2(6) = 68
According to Lewis-dot structure, there are 58 number of bonding electrons and 10 number of non-bonding electrons.
As we know that, single bond contains 1 sigma bond, double bond contains 1 sigma bond and 1 pi bond and triple bond contains 1 sigma bond and 2 pi bonds.
From the structure we conclude that, there are 18 sigma
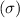 bonds and 4 pi
bonds and 4 pi
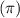 bonds.
bonds.