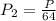Answer:
C).
Step-by-step explanation:
As we know if temperature of the gas remains constant then
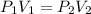
now we will have
P = initial pressure due to gas
initial volume of the gas is
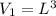
now we know that side of the box is increased by 4 times
so new volume is
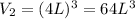
now we have
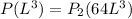
now we have