Answer:
Rate = 1.09*10^-3 (mol H2/L)/s
Step-by-step explanation:
Given:
Initial concentration of H2, C1 = 0 M
Final concentration of H2, C2 = 0.101 M
Time taken, t = 93.0 s
To determine:
The rate of the given reaction
Calculation:
The decomposition of PH3 is represented by the following chemical reaction
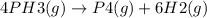
Reaction rate in terms of the appearance of H2 is given as:
![Rate = +(1)/(6)*(\Delta [H2]])/(\Delta t)](https://img.qammunity.org/2020/formulas/chemistry/college/u9hxaf748o33pd34kdr9oj4viggnt5oafa.png)
![Rate = +(1)/(6)*(C2[H2]-C1[H2])/(\Delta t)](https://img.qammunity.org/2020/formulas/chemistry/college/nfbwa9qga7e98hz0v4av5c75c7mf62vwgt.png)
Here C1(H2) = 0 M and C2(H2) = 0.101 M
Δt = 93.0 s
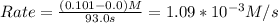
Since molarity M = mole/L
rate = 1.09*10^-3 (mol H2/L)/s