Answer: A. ethylene glycol (molar mass = 62.07 g/mol)
Step-by-step explanation:
Depression in freezing point is given by:
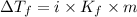
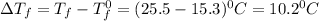 = Depression in freezing point
= Depression in freezing point
i= vant hoff factor = 1 (for non electrolyte)
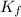 = freezing point constant =
= freezing point constant =
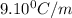
m= molality
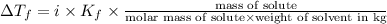
Weight of solvent (t-butyl alcohol)= 11.6 g = 0.0116 kg
Molar mass of unknown non electrolyte = M g/mol
Mass of unknown non electrolyte added = 0.807 g
![10.2=1* 9.10* (0.807g)/(M g/mol* 0.0116kg)[/tex ]</p><p>[texM=62.07g/mol](https://img.qammunity.org/2020/formulas/chemistry/high-school/75fpwblyg2yoqo0923lymant3rtjtvgu8w.png)
Thus the most likely the identity of this unknown liquid is ethylene glycol with molar mass of 62.07 g/mol.