Step-by-step explanation:
According to Arrhenius, acids are the species that dissociate in water to give a hydrogen ion or proton. And, bases are the species that dissociate in water to given hydroxide ions or
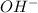 .
.
Bronsted-Lowry stated that acids are the species that donate a proton. And, bases are the species that accept a proton.
Lewis acid are the species that accept an electron pair. Whereas lewis bases are the species that donate an electron pair.
Therefore, given terms are matched with their definition as follows.
A). Arrhenius acid : Increases the
![[H^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/axnvi9gez4h3rovfp1qidd16ya8d7gzbon.png)
B). Arrhenius base : Increases the
![[OH^(-)]](https://img.qammunity.org/2020/formulas/chemistry/college/ug5fh4frhjslztec5ab506a86sstd1ikl9.png)
C). Brønsted-Lowry acid : Donates an
![[H^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/axnvi9gez4h3rovfp1qidd16ya8d7gzbon.png)
D). Brønsted-Lowry base : Accepts an
![[H^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/axnvi9gez4h3rovfp1qidd16ya8d7gzbon.png)
E). Lewis acid : Accepts an electron pair
F). Lewis base : Donates an electron pair