Answer : The number of moles of A remains are, 2.00 mole
Explanation :
As we are given that,
Number of moles of solid A = 4.80 mole
Volume of container = 1.00 L
Concentration of A =
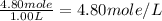
Concentration of B = 1.40 M
The moles of B in 1 liter of solution =
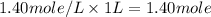
When the volume is doubled then we are using volume 2 L instead of 1 L.
The moles of B in 2 liter of solution =
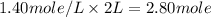
The balanced chemical reaction is,

initial conc. 4.80 0 0
At eqm. (4.80-2.80) 2.80
The moles of A remains = 4.80 - 2.80 = 2.00 mole
Therefore, the number of moles of A remains are, 2.00 mole