Answer:
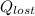 per mole of pentane = 3157.53 kJ/mol
per mole of pentane = 3157.53 kJ/mol
Step-by-step explanation:
Given:
Mass of pentane, m = 0.468 gram
Molar mass of pentane, M = 72.15
Now, mol of pentane, n = mass/M = 0.468/72.15 = 0.00648 mol of C5H12
Now,
ΔT = 23.65 - 20.45 = 3.2°C
Heat capacity of the calorimeter, C = 2.21 kJ/°C
Specific heat capacity of the water, Cp = 4.184 J/g.°C
Now,
the heat gained = the heat lost
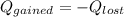
also,
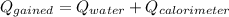
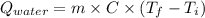
or
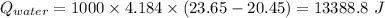
and
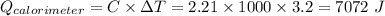
Now,
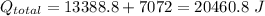
we have,
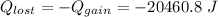 (Here negative sign depicts the release of the heat)
(Here negative sign depicts the release of the heat)
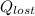 per mole of pentane =-20460.8/(0.00648 ) = 3157.53 kJ/mol
per mole of pentane =-20460.8/(0.00648 ) = 3157.53 kJ/mol