Answer:
The given compound cannot be cocaine.
Step-by-step explanation:
The chemist can comment on the nature of compound being cocaine or not from the depression in freezing point.
Depression in freezing point of is related to molality as:
Depression in freezing point = Kf X molality
Where
Kf = cryoscopic constant = 4.90°C/m
depression in freezing point = normal freezing point - freezing point of solution
depression in freezing point = 5.5-3.9 = 1.6°C
1.6°C = 4.90 X molality
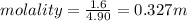
we know that:
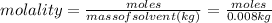
therefore
moles = 0.327X0.008 = 0.00261 mol
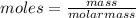
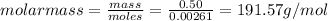
The molar mass of cocaine is 303.353
So the given compound cannot be cocaine.