Answer:
0.30042 kg = 300.42 gram
Step-by-step explanation:
Given data:
Density, D = 2.5 × 10³ kg/m³
Radius, r = 50 μm = 50 × 10⁻⁶ m³
edge of the cube, a = 1.1 m
Now, Mass (M) is given as :
M = Density × Volume
M =
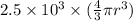
or
M =
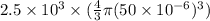
or
M = 1.30 × 10⁻⁹ Kg
Thus, the mass of single sand grain is 1.30 × 10⁻⁹ Kg
Now, the number of grains required for the 1.1 m edge cube total surface area
the surface area of the cube = number of sand grains (n) × (surface area of the single sand grain)
6 × a² = n × 4πr²
on substituting the values
we have
6 × 1.1² = n × 4 × π × (50 × 10⁻⁶)²
n = 231.092 × 10⁶
Thus, the mass of the sand required will be = n × M = 231.092 × 10⁶ × 1.30 × 10⁻⁹ Kg = 0.30042 kg = 300.42 gram