Step-by-step explanation:
The given data is as follows.
Surface area = 0.25
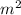 , current (I) = 25 A
, current (I) = 25 A
Density = 7.19 g/

As 1 m = 100 cm. Hence, 1
 =
=
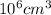
So, density =
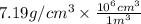
As Height = 0.01 mm =
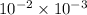 =
=
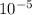 m.
m.
It is known that density is mass divided by volume.
Density =

Also, volume = Area × height. Therefore, substituting it into the above formula as follows.
Density =

Density =
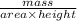
mass =
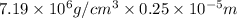
= 17.975 g
As number of moles equal mass divided by molar mass of the substance.
So, No. of moles =
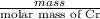
=
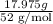
= 0.3457 mol
As there is transfer of total 6 electrons and it is known that charge on 1 mole of electron is 96500 C.
Hence, charge on 6 electrons will be as follows.
96500 C × 6 = 579000 C
This means that charge for 2 moles of
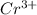 is 579000 C
is 579000 C
And, charge for 0.3457 mol of
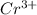 is
is
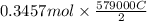 = 100080 C.
= 100080 C.
Also, Q =
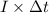
where, Q = charge
I = current
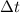 = time in seconds
= time in seconds
Now, putting the values into above formula as follows.
Q =
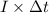
100080 C =
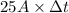
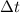 = 4003.2 sec
= 4003.2 sec
Converting seconds into hours as follows.
1 hr = 3600 sec
So,
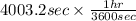
= 1.112 hr
Thus, we can conclude that time taken to apply chromium plating is 1.112 hr.