Answer:
Work out = 28.27 kJ/kg
Step-by-step explanation:
For R-134a, from the saturated tables at 800 kPa, we get
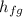 = 171.82 kJ/kg
= 171.82 kJ/kg
Therefore, at saturation pressure 140 kPa, saturation temperature is
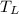 = -18.77°C = 254.23 K
= -18.77°C = 254.23 K
At saturation pressure 800 kPa, the saturation temperature is
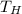 = 31.31°C = 304.31 K
= 31.31°C = 304.31 K
Now heat rejected will be same as enthalpy during vaporization since heat is rejected from saturated vapour state to saturated liquid state.
Thus,
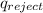 =
=
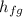 = 171.82 kJ/kg
= 171.82 kJ/kg
We know COP of heat pump
COP =
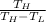
=
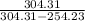
= 6.076
Therefore, Work out put, W =
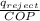
= 171.82 / 6.076
= 28.27 kJ/kg