Answer:
0.7M
Step-by-step explanation:
Volume of NaOH = 35mL = 0.035L
Concentration of NaOH = 0.5M
Volume of HCl = 25mL = 0.025L
Concentration of HCl = ?
Solution;
The balanced equation of the reaction is shown below:
NaOH + HCl → NaCl + H₂O
To solve this problem, we solve from the known to the unknow. The known here is the parameters of NaOH. From here, we can find the number of moles;
Number of moles of NaOH = concentration x volume= 0.035 x 0.5
= 0.0175mole
From the balanced reaction equation, we can state that:
1 mole of NaOH reacted with 1 mole of HCl
0.0175mole of NaOH will reacted with 0.0175mole of HCl
Now that we know the number of moles of the acid used, we can calculate the concentration of the acid used using the expression below:
Concentration of acid =
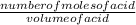
Concentration of acid=
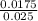 = 0.7M
= 0.7M