Given:
half life of
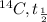 = 6000 years
= 6000 years
%quantity contained in sample,
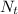 = 6.25%
= 6.25%
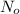 original quantity = 0.0625
original quantity = 0.0625
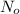
Formula used:
Formula for carbon dating is given by:
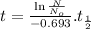
where,
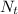 = amount of radio active material
= amount of radio active material
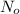 = amount of original material
= amount of original material
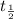 = half life
= half life
Solution:
Using the formula mentioned above with suitable values given:
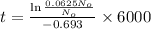
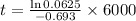
t = 24005.097 years
Therefore, the estimated age of the sample of the mummified skin is 24005.097 years