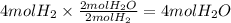Answer: The correct answer is
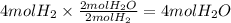
Step-by-step explanation:
We are given:
Moles of hydrogen gas = 4 moles
As, oxygen is given in excess. Thus, is considered as an excess reagent and hydrogen is considered as a limiting reagent because it limits the formation of products.
For the given chemical equation:
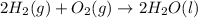
By Stoichiometry of the reaction:
2 moles of hydrogen produces 2 moles of water molecule.
So, 4 moles of hydrogen will produce =
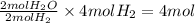 of water.
of water.
Hence, the correct answer is