Answer:
Wavelength,
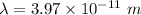
Step-by-step explanation:
It is given that,
Frequency of x - ray,

Let
 is the wavelength in vacuum of these X-rays. The relation between the wavelength and the frequency is given by :
is the wavelength in vacuum of these X-rays. The relation between the wavelength and the frequency is given by :
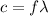
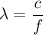
c is the speed of light
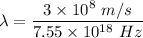
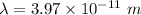
So, the wavelength in vacuum of these x-rays is
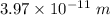 . Hence, this is the required solution.
. Hence, this is the required solution.