Answer:
A) The work done by the engine is: 6.8MJ/L
B) The fuel efficiency is
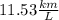
Step-by-step explanation:
A)
We know that the gasoline releases about 3.4*10^7 J of energy for each liter, and about 80% of that energy is lost as heat; it means that the other 20% of the energy released is taken for the engine to do work. In that sense, the work done by the engine is 20% of the 3.4*10^7 J that the gasoline releases for 1 liter, so:
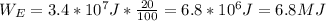
This last can be seen as a conversion factor, where we multiply the energy released by the gasoline by the factor (20 J taken for do work for each 100 J released).
B) We know that the car requires 5.9*10^5 J of work for each km traveled. That is the energy that the car requires, but it is not the energy that you have to give to the car; take in mind that the energy that you put in the car in gasoline liters will be not taken all, but just 20%. Also we know that the work done by the engine for 1 liter of gasoline is 6.8MJ, and that is just the work taken for do work (the useful energy), so we can connect both data:
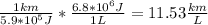
The first fraction,
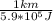 is the ratio or the proportion of (1 km requieres 5.9*10^5 J); and we multiply by the second fraction
is the ratio or the proportion of (1 km requieres 5.9*10^5 J); and we multiply by the second fraction
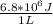 , which is the ratio: 6.8*10^6 J of work done for each liter of gasoline.
, which is the ratio: 6.8*10^6 J of work done for each liter of gasoline.