Answer:
ΔS = +541.3Jmol⁻¹K⁻¹
Step-by-step explanation:
Given parameters:
Standard Entropy of Fe₂O₃ = 90Jmol⁻¹K⁻¹
Standard Entropy of C = 5.7Jmol⁻¹K⁻¹
Standard Entropy of Fe = 27.2Jmol⁻¹K⁻¹
Standard Entropy of CO = 198Jmol⁻¹K⁻¹
To find the entropy change of the reaction, we first write a balanced reaction equation:
Fe₂O₃ + 3C → 2Fe + 3CO
To calculate the entropy change of the reaction we simply use the equation below:
ΔS = ∑S
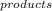 - ∑S
- ∑S
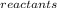
Therefore:
ΔS = [(2x27.2) + (3x198)] - [(90) + (3x5.7)] = 648.4 - 107.1
ΔS = +541.3Jmol⁻¹K⁻¹