Answer:
15.36 moles of carbon dioxide are produced when 5.12 mol of propane gas is burned in excess oxygen
Step-by-step explanation:
The Balanced Chemical equation is :
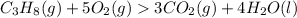
Mole ratio of
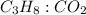 is 1 : 3
is 1 : 3
That is, 1 mole of propane gas produces 3 moles of carbon dioxide gas.
Using mole ratio we can solve this
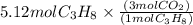
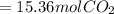 is produced.
is produced.
(Answer)
Please note :
To convert moles to mass, we multiply by molar mass
To convert mass to moles, we divide by molar mass
Molar mass is the mass of 1 mole of the substance
For example molar mass of
 is
is
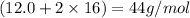
(we just add the atomic mass of the atoms to get the molar mass of the substance)