Answer:
Option (A) 50 °C
Step-by-step explanation:
Thinking process:
Let the volume of the hot air be
 5.30 k l = 5 300 l = 5.3 m³
5.30 k l = 5 300 l = 5.3 m³
The initial temperature will be 12 ° (12 + 273.15) = 285.15 K
The later volume,
 = 6 Kl = 6 000 l = 6 m³
= 6 Kl = 6 000 l = 6 m³
The equation will be:
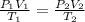
If the pressure is the same, then:
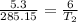
Solving for
 gives:
gives:
T₂ = 322.8
= 49.66
= 50°