Answer:
(C) 503 g of water, H2O, are produced from 317 grams of ammonia and excess oxygen
Step-by-step explanation:
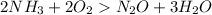
Molar mass of
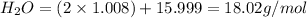
Molar mass of
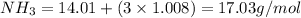
The conversions are
Step 1:
Mass
 to moles
to moles
 by dividing with molar mass
by dividing with molar mass

Step 2:
Moles
 to moles
to moles
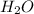 by using mole ratio of
by using mole ratio of
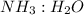 i.e., 2 : 3
i.e., 2 : 3
Step 3:
Moles
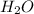 to mass
to mass
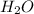 by multiplying with molar mass
by multiplying with molar mass
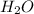
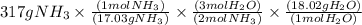
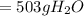 is formed.
is formed.
(Answer)
Please note :
To convert moles to mass, we multiply by molar mass
To convert mass to moles, we divide by molar mass
Molar mass is the mass of 1 mole of the substance
For example molar mass of
 is
is
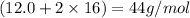
(we just add the atomic mass of the atoms to get the molar mass of the substance).