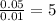Answer:
K = 5
Step-by-step explanation:
Partial coefficient is also known as distribution coefficient, which is the ratio of concentration of a solute in two immiscible liquids.
For given system we write it as:
Kd =
![([salicylicacid]_(dichloromethane))/([salicylicacid]_(water))](https://img.qammunity.org/2020/formulas/chemistry/high-school/9ofpf2h63iexikm06nrrmqhnyiz5qbyxjc.png)
After extraction the concentration of salicylic acid in dichloromethane is 1g /20 mL = 0.05
Initially the amount of salicyclic acid present was = 2 g
out of this one grams was being extracted by organic solvent
The amount of solute remained in aqueous phase = 1 g
so the concentration of salicyclic acid in water = 1/ 100 = 0.01
Kd =