(a)
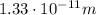
The x-rays in the tube are emitted as a result of the collisions of electrons (accelerated through the potential difference applied) on the metal target. Therefore, all the energy of the accelerated electron is converted into energy of the emitted photon:
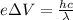
where the term on the left is the electric potential energy given by the electron, and the term on the right is the energy of the emitted photon, and where:
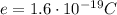 is the electron's charge
is the electron's charge
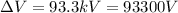 is the potential difference
is the potential difference
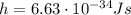 is the Planck constant
is the Planck constant
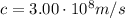 is the speed of light
is the speed of light
 is the wavelength of the emitted photon
is the wavelength of the emitted photon
Solving the formula for
 , we find:
, we find:
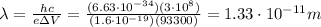
(b) 93300 eV (93.3 keV)
The energy of the emitted photon is given by:

where
h is Planck constant
c is the speed of light
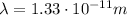 is the wavelength of the photon, calculated previously
is the wavelength of the photon, calculated previously
Substituting,
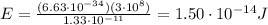
Now if we want to convert into electronvolts, we have to divide by the charge of the electron:
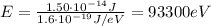
(c) The following statements are correct:
The maximum photon energy is just the applied voltage times the electron charge. (1)
The value of the voltage in volts equals the value of the maximum photon energy in electron volts.
In fact, we see that statement (1) corresponds to the equation that we wrote in part (a):
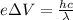
While statement (2) is also true, since in part (b) we found that the photon energy is 93.3 keV, while the voltage was 93.3 kV.