Answer:
1711.11L
Step-by-step explanation:
Given parameters:
Mass of methane = 1kg = 1000
Temperature of gas = 35°C
Pressure of the gas = 0.9atm
Converting to the temperature to kelvin, we have:
35+ 273 = 308k
Solution
From the given parameters, we can solve this problem using the ideal gas equation which is combination of the gas laws. It is expressed below:
PV = nRT .......................(i)
Where P is the pressure of the gas
V is the volume of the gas
n is the number of moles
R is the gas constant
T is the temperature
Now, from the above parameters, we have two unknowns which are n and V.
To find the number of moles n, we use the expression below:
number of moles =
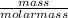
molar mass of methane = 12 + (4x1) = 16g/mol
Number of moels of methane =
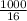 = 62.5mole
= 62.5mole
Now that we know the number of moles, we can make the unknown Volume the subject of the formula:
V =
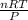
V =
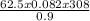 = 1711.11L
= 1711.11L