Answer: D. its chemical properties
Step-by-step explanation:
Chemical property is defined as the property of a substance which becomes evident during chemical change in which a change in chemical composition takes place. A new substance is formed in these reactions. Example: Reactivity of hydrogen with oxygen
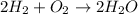
Physical property is defined as the property of a substance which becomes evident during physical change in which there is alteration in shape, size etc. No new substance gets formed during physical change. Example: Fusion of ice
Thus property of matter that determine how that substance will react when combined with other substances is its chemical properties.