Answer:
3.7 atm i.e., pressure doubles
Step-by-step explanation:
P₁ = Initial pressure = 1.85 atm
P₂ = Final pressure
V₁ = Initial volume = 12.5 L
V₂ = Final volume = 0.5V₁
T = Temperature is constant
From ideal gas law
P₁V₁ = P₂V₂
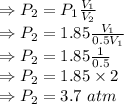
∴ Final pressure is 3.7 atm i.e., pressure doubles