Answer:
concentration = 0.044 moles / L
Step-by-step explanation:
The mass of non electrolyte solute dissolved in water =4.96 grams
The volume of water taken = 485mL = 0.485 L
the temperature = 27°C = 300 K
osmotic pressure = 827 torr =
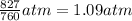
The osmotic pressure is related to molar concentration as:
osmotic pressure = concentration X R X T
Where
R = gas constant = 0.0821 L atm/molK
Putting values
1.09 = concentration X 0.0821 X 300
concentration = 0.044 moles / L