Step-by-step explanation:
1) Formulas of the reactants:
Hydrogen chloride : HCL
Sodium sulfide :
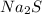
2) Formulas of the products:
Sodium chloride : NaCl
Hydrogen sulfide :
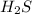
3) Balance chemical equation is given as:
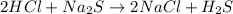
2 moles of hydrogen chloride reacts with 1 mole of sodium sulfide to produce 1 mole of hydrogen sulfide along with 2 moles sodium chloride.