Answer:
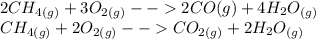
Step-by-step explanation:
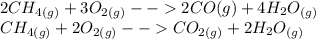
The incomplete combustion of alkanes, and other fuels actually, happens when there is a limited supply of oxygen. Instead of the fuel burning completely to produce carbon dioxide, it produces carbon monoxide instead.
This gas is harmful to jumans because it combines with haemoglobin in lood and takes up space that belongs to oxygen which can lead to suffocation or even death