Answer:
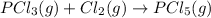
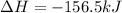
Step-by-step explanation: It's based on Hess's law. We will rearrange the two given equations and make the equation to which it asks to calculate the enthalpy change.
First equation needs to be reversed and divided by 2 so that we could get one
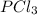 on reactant side. When we reversed an equation then the sign of enthalpy change is also changed.
on reactant side. When we reversed an equation then the sign of enthalpy change is also changed.
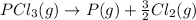
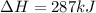
Second equation also needs to be divided by 2 and its not reversed.
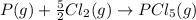
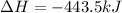
Both the equations are added now:
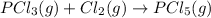
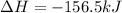
So, the enthalpy change for the equation is -156.5 kJ.