Answer : The molarity and molality of the solution is, 18.29 mole/L and 499.59 mole/Kg respectively.
Solution : Given,
Density of solution =
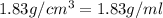
Molar mass of sulfuric acid (solute) = 98.079 g/mole
98.0 % sulfuric acid by mass means that 98.0 gram of sulfuric acid is present in 100 g of solution.
Mass of sulfuric acid (solute) = 98.0 g
Mass of solution = 100 g
Mass of solvent = Mass of solution - Mass of solute = 100 - 98.0 = 2 g
First we have to calculate the volume of solution.
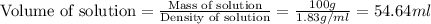
Now we have to calculate the molarity of solution.
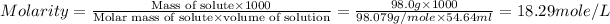
Now we have to calculate the molality of the solution.
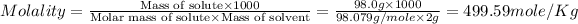
Therefore, the molarity and molality of the solution is, 18.29 mole/L and 499.59 mole/Kg respectively.